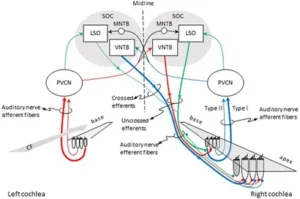
Figure 1. Pathways for activation of medial (MOC) and lateral olivocochlear (LOC) efferent fibers to the right cochlea. Red and blue lines illustrate the pathways for activation of the contralateral and ipsilateral MOC reflexes, respectively. Green lines illustrate the pathways for activation of the LOC reflex. The thickness of each line roughly illustrates the density of innervation. Abbreviations: LSO, lateral superior olive; MNTB, medial nucleus of the trapezoid body; PVCN, postero-ventral cochlear nucleus; SOC, superior olivary complex; VNTB, ventral nucleus of the trapezoid body; CF, characteristic frequency.
https://www.frontiersin.org/journals/neurology/articles/10.3389/fneur.2018.00197/full
https://www.sciencedirect.com/topics/medicine-and-dentistry/olivocochlear-system
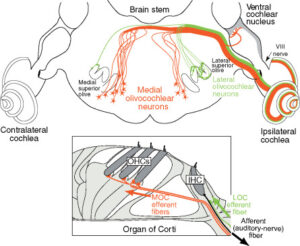
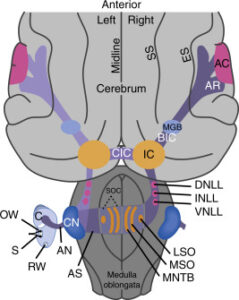
Figure 1. Neural pathways of the medial and lateral olivocochlear neurons in the brain stem and cochlea (inset). Only the efferent pathways to the right (ipsilateral) cochlea are illustrated. Large arrows indicate the direction of spike transmission. An afferent auditory-nerve fiber (type I) from an inner hair cell is illustrated in black; auditory nerve fibers (type II) from outer hair cells are not illustrated. Adapted from Liberman MC (1990) Effects of chronic cochlear de-efferentation on auditory-nerve response. Hearing Research 49: 209–224, with permission from Elsevier.
Hair cell receptors within the auditory, vestibular, and lateral line systems receive an abundant efferent innervation from the central nervous system. This characteristic sets them apart from receptors in other sensory modalities. The auditory receptors receive innervation from the olivocochlear (OC) system, named because of its origin in the brain stem’s superior olivary complex and termination in the cochlea (inner ear) (Figure 1). Two separate systems of OC neurons exist. Lateral olivocochlear (LOC) neurons originate in and near the lateral superior olive and project to auditory-nerve dendrites beneath the inner hair cells. Medial olivocochlear (MOC) neurons originate in the more medial parts of the complex and project to outer hair cells (Figure 1). LOC neurons are located predominantly on the same side of the brain as the cochlea that they innervate, whereas MOC neurons are distributed bilaterally
Pictures
https://images.app.goo.gl/jASADXctVpAe3Fss6
”The Olivocochlear System: It has been known for more than 50 years that the olivocochlear bundle (Figs. 2, 29 and 32) provides the organ of Corti with efferent innervation (Rasmussen, 1946, 1953). It should be noted that the auditory-vestibular system appears unique among sensory systems because hair cell receptors receive direct projections from the brain (Robertson, 2009). Two systems of olivocochlear neurons are recognized, the medial (MOC) and lateral (LOC) systems (Figs. 29 and 32; White and Warr, 1983; Warr et al., 1986, 1997; Spangler and Warr, 1991; Warr, 1992; Vetter et al., 1991; Vetter and Mugnaini, 1992; Cantos et al., 2000). As their names imply, their respective cell bodies are located in medial and lateral regions of the SOC.
The MOC neurons innervate the OHCs (Figs. 2, 29 and 32). About 55% of them originate on the contralateral side and the reminder on the ipsilateral side (rat: Robertson et al., 1989; Horváth et al., 2000). The projection is made up of thick myelinated axons that terminate directly on the OHCs (White and Warr, 1983; Warr et al., 1997; Horváth et al., 2000). MOC neurons (Fig. 32) generally have large cell bodies that are stellate- or triangular-shaped, and they possess tapering dendritic arbors that radiate and branch profusely (Osen et al., 1984; Vetter and Mugnaini, 1992). They are located ventral to the MSO, lateral to MTz, and in the VTz (Fig. 29; Vetter and Mugnaini, 1992) and constitute a neurochemically homogeneous population of cholinergic cells (Osen et al., 1984; Vetter et al., 1991). They may receive descending input from the ipsilateral IC (see above, Vetter et al., 1993) and ascending input bilaterally from the VCP and also possibly from the globular bushy cells in the caudal VC, as White (1986) found terminal boutons similar to the calyces of Held terminating upon MOC cells. Collaterals of the axons of MOC neurons innervate VC neurons (Fig. 29; Hováth et al., 2000). The projections to the cochlea innervate clusters of OHC spanning as much as an octave of cochlear length, but the terminal axonal arbor is often centered basal to the corresponding location of radial afferent fibers of similar best frequency. MOC neurons are sharply tuned and the MOC efferent innervation is tonotopically organized (reviewed in Warr, 1992; Guinan, 1996; Helfert and Aschoff, 1997).
LOC neurons innervate the type I auditory nerve fibers that receive input from the IHCs. They originate predominantly on the ipsilateral side of the brain (Figs. 29 and 32; rat: White and Warr, 1983; Warr et al., 1997; Horváth et al., 2000; cat: Arnesen and Osen, 1984; Liberman and Brown, 1986; mouse: Brown, 1987, Wilson et al., 1991; Brown and Levine, 2008; guinea pig: Brown, 1993). In rats, two distinct types of neurons form the LOC, intrinsic and shell neurons (Fig. 32; Vetter and Mugnaini, 1992; Warr et al., 1997). All intrinsic neurons (Figs. 29 and 32) are small cells confined within the limits of the ipsilateral LSO. They possess a disc-shaped dendritic arbor, similar to that of the LSO principal neurons, with thin untapered dendrites and constitute about 85% of all LOC neurons (Vetter and Mugnaini, 1992; Warr et al., 1997; Horváth et al., 2000).
The intrinsic neurons probably receive the same input as the principal cells in the LSO. About 50% of them are GABAergic and the remaining 50% that are cholinergic, co-localize calcitonin gene-related peptide (Vetter et al., 1991). Their axons are thin and unmyelinated (being 0.77 μm thick in the organ of Corti), travel relatively short distances in the inner spiral bundle and terminate forming discrete (less than 0.2 octave in extent) terminal arborizations with a focal, tonotopic organization (Warr et al., 1997). They do not innervate the VC (Horváth et al., 2000). Most shell neurons (Figs. 29 and 32; about 95%) are located in areas surrounding the ipsilateral LSO. They are large multipolar cells with thick and tapered dendrites and constitute about 15% of the LOC cells (Vetter and Mugnaini, 1992; Warr et al., 1997). Little is known about the source of the inputs they receive. Only one-third of these shell neurons is GABAergic, while the other two-thirds are cholinergic but do not co-localize calcitonin gene-related peptide (Vetter et al., 1991). Their axons are thin and unmyelinated (being 0.37 μm thick in the organ of Corti), travel long distances (greater than 1 mm) in the inner spiral bundle and terminate forming diffuse (greater than 1 octave in extent) terminal arborizations; they are probably not tonotopic (Warr et al., 1997). In contrast to the intrinsic neurons, but similar to MOC, they innervate the VC (Figs. 2, 29; Horváth et al., 2000).
Physiological studies in several species have shown that MOC neurons are sharply tuned and have a wide dynamic range. Most of them respond to binaural stimuli. Two-thirds to contralateral stimuli and one-third to ipsilateral stimuli (Liberman, 1988; reviewed in Guinan, 1996). Stimulation of MOC neurons is thought to raise the threshold of the primary afferent fibers through the modulation of the biomechanical properties of the cochlear duct. MOC neurons may enhance transduction or signal detection through an unmasking effect, thus regulating the slow motility of the OHCs and thereby the stiffness of the basilar membrane (reviewed in Helfert and Aschoff, 1997; Eggermont, 2001). Previous studies have also demonstrated that the MOC can somewhat reduce the temporary threshold shift that occurs as an early manifestation of the damaging effects of loud sounds (guinea pig: Roberston and Johnstone, 1980; Cody, 1992; reviewed in Guinan, 1996), protecting the cochlea from acoustic injury (Taranda et al., 2009). Moreover, after cochlear dysfunction, MOC neurons seem to be a major source of synaptic reorganization in the VC that could possibly entail compensatory activation of the affected ascending auditory pathway (Kraus and Illing, 2004). The functional role of the LOC is still uncertain, but a recent study in mice has shown that lateral olivocochlear feedback maintains the binaural balance in neural excitability required for accurate localization of sounds in space (Darrow et al., 2006). In addition, several immunocytochemical studies have shown that these neurons contain neuroactive substances such as dopamine, serotonin and opioid peptides (e.g., Safieddine and Eybalin, 1992; Eybalin, 1993; Safieddine, et al., 1997; Inoue et al., 2006), which speaks in favor of a modulatory effect on the afferent fibers that innervate the IHCs. Rajan (2000) has shown in cats that even moderate background noise can significantly exacerbate the cochlear temporary threshold shift induced by loud tones, but that this is prevented when there is additional activation of the LOC. Thus, in background noise there is a conjoint activation of the MOC and LOC to powerfully protect (by almost 30 dB) the cochlea from loud sounds that would otherwise be exacerbated by the noise (Rajan, 2000). Clearly, the olivocochlear system plays a critical role in maintaining the normal operations of the cochlea. The presence of efferent connection with feedback loops grateful may introduce non-linear dynamics (Eggermont, 2001) into the auditory system.
https://www.sciencedirect.com/topics/medicine-and-dentistry/olivocochlear-system
https://images.app.goo.gl/jASADXctVpAe3Fss6
https://www.google.com/url?sa=i&url=https%3A%2F%2Fwww.sciencedirect.com%2Fscience%2Farticle%2Fpii%2FS0378595521000411&psig=AOvVaw0p6I4cGGBIIa3liN6LMTzE&ust=1727786453480000&source=images&cd=vfe&opi=89978449&ved=0CBEQjRxqFwoTCLDNnojZ6ogDFQAAAAAdAAAAABAE

Figure 1. Illustration of the olivocochlear (OC) anatomy. Three different groups of efferent neurons have been identified in the superior olivary complex: (1) intrinsic lateral olivocochlear (LOC) neurons (green), (2) shell neurons from the lateral superior olive (light blue), and (3) medial olivocochlear (MOC) neurons from the medial superior olive (orange). MOC neurons have thick and myelinated axons that mainly project to the contralateral cochlea and make synapses with OHCs. LOC neurons and shell neurons project mainly to the ipsilateral cochlea and make synapses with afferent auditory-nerve fibers. Shell neurons originate in the periphery of the LOC nuclei, and their projections bifurcate to the base and apex of the cochlea. LOC intrinsic neurons are the largest component of the OC system and emerge from the LOC nuclei themselves. LOC axons project unidirectionally to the apex or base of the cochlea. Neurons located in the LOC medial limb project to the base of the cochlea (H: high frequencies), whereas those located in the lateral limb project to the apex (L: low frequencies). Differences in the distribution of OC neurons among mammals can be found in Table 1 (OHC: outer hair cell; IHC: inner hair cell).
These two bundles of OC efferents, besides their different origin, project to different targets in the cochlea. The MOC fibers project mostly contralaterally, making synaptic contact with the three rows of outer hair cells (OHCs), while LOC fibers project mostly ipsilaterally, making synaptic contact with radial afferent auditory-nerve fibers beneath inner hair cells (IHCs; Guinan, J. J., Jr., 1996).
In the following sections the anatomy and neurotransmitters of the medial and lateral OC systems are reviewed separately. For further information in the anatomy and physiology of these systems see Guinan J. J., Jr. (1996) or in cochlear neurotransmitters see Eybalin M. (1993).
3.24.2.1.1 Medial olivocochlear anatomy
MOC fibers emerge from the ventral nucleus of the trapezoid body, and in some species (cats and guinea-pigs) also from the superior periolivary nucleus. In the mature mammalian cochlea, MOC fibers make direct synaptic contact only with OHCs; however, during development, immature IHCs also receive transient innervation from MOC efferents that disappears in later developmental stages (Simmons, D. D., 2002). Although there are differences in the distribution of efferent fibers among species, in all of those studied, most of the MOC fibers cross to the contralateral cochlea through the floor of the fourth ventricle (Table 1). In cats, crossed MOC fibers project mainly to the more basal turns of the cochlea, while uncrossed MOC projections are largest in the center of the cochlea (Guinan, J. J., Jr., 1996). In contrast, the largest MOC projections in mice are directed toward the middle of the cochlea, a location that in this species corresponds to characteristic frequencies around 10 kHz (Maison, S. F. et al., 2003a). Another species-specific difference in efferent innervation is that the MOC synaptic terminal area in cats is four times larger for the first row than that for the third row of OHCs (Liberman, M. C. et al., 1990), whereas in mice there is no gradient of MOC innervation across OHC rows (Maison, S. F. et al., 2003a). In addition to their well-studied cochlear projections, MOC neurons in various species also send axon collaterals to the ventral cochlear nucleus and the vestibular nuclei (Brown, M. C. et al., 1991; Brown, M. C., 1993; Horvath, M. et al., 2000).
Table 1. Species differences in the distribution of olivocochlear efferent fibers
| Species | Medial olivocochlear system (MOC) | Lateral olivocochlear system (LOC) | ||||
|---|---|---|---|---|---|---|
| Intrinsic LOC | Shell LOC | |||||
| Number of neurons | Percentage of contralateral fibers | Number of neurons | Percentage of ipsilateral fibers | Number of neurons | Percentage of ipsiateral fibers | |
| Chinchilla (Azeredo, W. J., et al., 1999) | 280 | 80% contralateral | 787 | 99% ipsilateral | 101 | 70% ipsilateral |
| Rat (Vetter, D. E. and Mugnaini, E., 1992) | 316 | 60% contralateral | 619 | 100% ipsilateral | 111 | 95% ipsilateral |
| Cat (Warr, W. B., et al., 2002) | 549 | 75% contralateral | 769 | 87% ipsilateral | 251 | 75% ipsilateral |
| Hamster (Sanchez-Gonzalez, M. A., et al., 2003) | 80 | 75% contralateral | 164 | 97% ipsilateral | 65 | 80% ipsilateral |
| Guinea-pig (Robertson, D., et al., 1987) | 543 | 69% contralateral | 690 LOC fibers (99% ipsilateral) | |||
| Mouse (Campbell, J. P. and Henson, M. M., 1988) | 164 | 75% contralateral | 311 LOC fibers (99% ipsilateral) (around 30 shell neurons, suggested by Darrow, K. N. et al., 2006a) | |||
| Human (Arnesen, A. R., 1984) | 360 myelinated fibers (MOC) | 1005 unmyelinated fibers (LOC) | ||||
An analysis of the anatomy of single MOC neurons performed in selectively labeled axons in rats demonstrated great morphological diversity of MOC neurons, which was evident at the organ of Corti in the number of tunnel-crossing fibers (1–15) and of synaptic boutons contacting OHCs (Warr,W. B. and Boche, J. E., 2003). According to their pattern of axonal branching within the cochlea, MOC neurons were sorted into two subtypes: a more common sparsely branched and a quite rare highly branched. In the limited sample of MOC axons analyzed in that study all highly branched fibers projected ipsilaterally and innervated short spans of the cochlea.
3.24.2.1.2 Lateral olivocochlear anatomy
The unmyelinated axons of the lateral OC system originate from two subgroups of efferent brainstem neurons: intrinsic and shell LOC neurons (Figure 1; Vetter, D. E. and Mugnaini, E., 1992). The intrinsic LOC neurons are small neurons located within the lateral superior olivary nucleus that project almost exclusively to the ipsilateral cochlea and have dendrites restricted to the nucleus. Intrinsic neurons have a coarse tonotopic distribution in the lateral superior olive (LSO); as neurons located in the lateral limb project to the cochlear apex and those located in the medial limb project to the base (Robertson, D. et al., 1987). In contrast with the intrinsic neurons, shell LOC neurons are a small group of large neurons that surround the LSO and project mainly to the ipsilateral cochlea (Vetter, D. E. and Mugnaini, E., 1992). Shell neurons have extensive dendrites that reach into the lateral superior olivary nucleus and probably make contact with other brainstem nuclei. In the rat, shell neurons, like MOC neurons, send axon collaterals that innervate the ventral cochlear nucleus, whereas intrinsic LOC neurons do not project into the ventral cochlear nucleus (Horvath, M. et al., 2000).
Based upon the patterns of cochlear innervation in the guinea-pig, LOC axons have been classified into two groups (Brown, M. C., 1987). The largest group of LOC fibers runs unidirectionally (either apically or basally), usually less than 1 mm, in a spiral path within the cochlea and make discrete terminations with synaptic contacts beneath IHCs, while the smallest group of fibers runs bidirectionally in the cochlea, forming axonal swellings throughout a more extended spiral course. A later study also found two types of LOC axons in the rat and showed that axons with focal terminal patterns beneath IHCs corresponded to intrinsic neurons, whereas those with diffuse terminal patterns corresponded to shell neurons (Warr, W. B. et al., 1997; Warr, W. B. and Boche, J. E., 2003). Although, it is known that lateral OC fibers make synaptic contacts with the dendrites of afferent auditory neurons, just beneath their synapses with the IHCs, it is not clear yet whether these efferent synapses correspond to either one or the other or to both types of LOC axons. Lateral efferents have also been reported to make sporadically synapses directly onto the IHCs (Liberman, M. C., 1980; Liberman, M. C. et al., 1990).
3.24.2.1.3 Medial olivocochlear neurotransmitters
It is well established that the main neurotransmitter of the MOC efferent system, that makes synaptic contact onto OHCs, is acetylcholine (ACh; Eybalin, M. 1993). In some species, there is also evidence that a subset of MOC neurons release gamma-aminobutyric acid (GABA) and calcitonin gene related peptide (CGRP) (Raphael, Y. and Altschuler, R. A., 2003).
Acetylcholine activates a nicotinic receptor located in the basolateral membrane of the OHCs, which has only recently been identified. Elgoyhen A. B. et al., 1994 and collaborators isolated and characterized α9, a new member of the nicotinic ACh receptor subunit gene family, which injected in Xenopus oocytes expressed a homomeric receptor-channel complex. This ion channel, as the other members of the family, is permeable to Na+ and Ca2+ and is activated by acetylcholine. The α9 nicotinic receptor displays unusual pharmacological properties, as it is blocked by nicotinic as well as muscarinic antagonists. In situ hybridization studies in the rat show that the α9 nicotinic receptor is expressed in only a few regions of the brain and in the hair cells of the cochlea, both in IHCs and OHCs. Based on this information, the α9 nicotinic receptor was postulated as a candidate for efferent transmission in the cochlea. However, there were still some differences between the biophysical properties of the heterologous expressed homomeric α9 nicotinic receptor and those of the native cholinergic receptor from isolated OHCs. In a later study, Elgoyhen A. B. et al. (2001) found another α nicotinic subunit, the α10, which in the adult rat is only expressed in OHCs. Their data show that a heteromeric α9/α10 nicotinic ACh receptor presents pharmacological and biophysical properties indistinguishable from those of the native cochlear hair-cell receptor. Recent studies in a heterologous expression of α9 and α10 subunits with different mutations in Xenopus oocytes (Plazas, P. V. et al., 2005) suggest that the native α9/α10 nicotinic receptor is constituted by a pentamer with a (α9)2(α10)3 stoichiometry (Figure 2). For a review in nicotinic acetylcholine receptors in the auditory efferent system, see Lustig L. R. (2006).

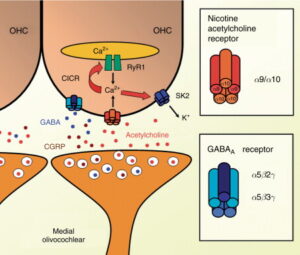
Figure 2. Medial olivocochlear (MOC) synapses with OHCs. The main neurotransmitter released by MOC fibers is acetylcholine that in mice co-localizes with GABA and CGRP (Maison, S. F. et al., 2003a). The opening of α9/α10 acetylcholine receptors generates an inward Ca2+ current in OHCs. An outward current through small-conductance Ca2+-activated K+ channels (SK2) hyperpolarizes OHCs. It is also postulated that the opening of acetylcholine receptors produces a Ca2+-induced Ca2+ release (CICR) from an intracellular cistern through ryanodine receptors (RyR 1). Inset panels on the right show the putative conformations of the pentameric α9/α10 acetylcholine receptor, and of the GABA receptor at the mouse cochlea (Plazas, P. V. et al., 2005; Maison, S. F. et al., 2006). OHC, outer hair cell.
There is evidence in mice and guinea-pigs that a subset of MOC fibers releases GABA (Fex, J. et al., 1986; Maison, S. F. et al., 2003a) and CGRP (Safieddine, S. and Eybalin, M., 1992). The presence of GABA and glutamic acid decarboxylase in efferent endings has been shown by inmunocytochemistry, and GABAA receptors have also been detected in the basolateral domain of OHCs by monoclonal antibodies against α and β subunits of the GABAA receptor (Plinkert, P. K. et al., 1989; 1993; see also Eybalin, M., 1993). These results, obtained in the guinea-pig cochlea, show a tonotopical distribution of gabaergic fibers and GABAA receptors, with a predominant presence in the upper cochlear turns. This tonotopical arrangement of gabaergic efferent fibers seems to be opposite to that of cholinergic fibers which are primarily located in the more basal regions of the cochlea (Plinkert, P. K. et al., 1993). In contrast, in mice OHC efferent innervation presents a density peak, similar for acetylcholine, GABA, and CGRP markers, in the central cochlear region (near 10 kHz) and lower values of density in the apical and basal regions (Maison, S. F. et al., 2003a). Furthermore, in mice near 100% co-localization between the three neurotransmitters has been found (Maison, S. F. et al., 2003a), whereas in guinea-pigs only a subset of MOC cholinergic fibers co-localizes for ACh and CGRP (Safieddine, S. and Eybalin, M., 1992) and another subset co-localizes for ACh and GABA (Fex, J. et al., 1986).
3.24.2.1.4 Lateral olivocochlear neurotransmitters
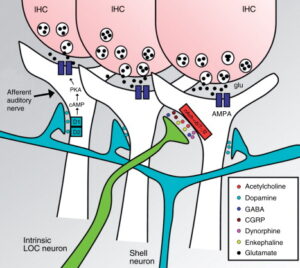
Several neurotransmitters and neuromodulators are known to be released by the LOC system: Acethylcholine, GABA, dopamine, dynorphines, enkephalin, and CGRP (Eybalin, M., 1993), and some of them have been shown to co-localize in LOC neurons. An early work demonstrated in cats that most LOC neurons stained with retrograde tracers (injected into the cochlea), also co-stained with acetylcholinesterase (AChE) (Warr, W. B., 1975). Another work using inmunohistochemistry, in cats and guinea-pigs, found that lateral efferent fibers beneath IHCs exhibited enkephaline-like reactivity (Fex, J. and Altschuler, R. A., 1981). Later, by means of double and triple inmunofluorescence co-localization techniques it was demonstrated, in guinea-pigs and rats, the presence of extensive co-localization of ACh, dynorphines and enkephalines (Abou-Madi, L. et al., 1987) and of ACh, GABA, dopamine, CGRP and enkephaline in most lateral efferent neurons (Safieddine, S. et al., 1997). In contrast, Darrow K. N. et al. (2006a) using inmunostaining for tyrosine hydroxylase (dopamine marker) and vesicular acetylcholine transporter in mouse cochleae showed little co-localization of both neurotransmitters in axon terminals beneath IHCs. Consistently, they found that in the brainstem most dopaminergic neurons are distinct from cholinergic neurons (Figure 3). Their results also suggested that in mice the bulk of the lateral efferent fibers reaching the cochlea are cholinergic and only 10–20% of them are dopaminergic (tyrosine-hydroxylase positive).
Figure 3. Lateral olivocochlear synapses with auditory-nerve fibers. Neurotransmitters released by lateral olivocochlear (LOC) fibers include: acetylcholine, dopamine, GABA, CGRP, dynorphines, and enkephalines. The main neurotransmitter between IHCs and auditory-nerve fibers is glutamate. In mice, LOC dopaminergic fibers originate from shell neurons (light blue) and are a separate group from LOC intrinsic fibers (green) that release acetylcholine and other neurotransmitters (Darrow, K. N. et al., 2006a). The dopamine receptors in the auditory-nerve afferent fiber (D1/D2) through a G-protein regulate the intracellular concentration of cAMP and thus the activity of the protein kinase A (PKA), which phosphorilates AMPA glutamate-receptors (Niu, X. and Canlon, B. 2006). Acetylcholine released by LOC fibers is thought to activate receptors conformed by α6, α7, and β2 subunits (Morley, B. J. et al., 1998). IHC, inner hair cell.
Acetylcholine released by OC lateral efferents probably activates cholinergic receptors composed by α6, α7, and β2 subunits (Morley, B. J. et al., 1998) that depolarize afferent auditory-nerve fibers and enhance glutamatergic transmission from IHCs (Felix, D. and Ehrenberger, K., 1992). Recent studies using inmunocytochemistry in guinea-pig cochlea have established the presence of dopamine transporters in the lateral efferent fibers below IHCs and in the inner spiral bundle (Ruel, J. et al., 2006) and also the presence of D1 receptors in the spiral ganglion neurons and in the afferent nerve endings beneath IHC synapses (Niu, X. and Canlon B., 2006).
In the brainstem, dopaminergic neurons are mainly located surrounding the LSO and they probably correspond to the shell neurons (Vetter, D. E. and Mugnaini, E., 1992), while AChE stained neurons are located within the LSO nucleus, corresponding to LOC intrinsic neurons (Darrow, K. N. et al., 2006a). In a recent study combining intracochlear injections of a fluorescent retrograde tracer and inmunofluorescent staining of tyrosine hydroxylase in the guinea-pig, Mulders W. H. and Robertson D. (2004) succeeded in demonstrating that dopaminergic neurons from the LSO project to the cochlea through the lateral efferent fibers. In agreement with previous observations (Safieddine, S. et al., 1997), they also found that dopaminergic neurons are not equally distributed over the LSO. Most of them originate from the medial limb, the region of the LSO that receives high-frequency input. Consistently, they found dense tyrosine-hydroxylase labeling in the first and second cochlear turns and sparse or no labeling in the apical cochlear regions. These results are in contrast with evidence that shows no differences in the distribution of D1 receptors beneath IHCs along the guinea-pig cochlea (Niu, X. and Canlon, B., 2006) and a uniform dopaminergic innervation from base to apex in the mouse cochlea (Darrow, K. N. et al., 2006a). It has been suggested that dopaminergic innervation may be important for high frequencies in the mammalian cochlea. This fact would explain the presence of dopaminergic fibers only in the basal turns of the cochlea in guinea-pig and through the whole cochlea in the mouse, which is a high-frequency mammal.
More?
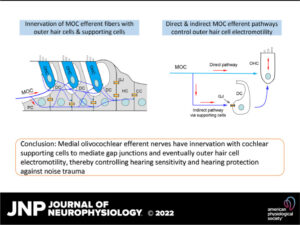
Efferent neurons control hearing sensitivity and protect hearing from noise through the regulation of gap junctions between cochlear supporting cells
https://pmc.ncbi.nlm.nih.gov/articles/PMC8759971/
ATP-styrda P2x7-receptorer uttrycks vid typ II-hörselnerver och krävs för efferent hörselkontroll och bullerskydd – PubMed TINNITUS HYPERACUSIA
https://pubmed.ncbi.nlm.nih.gov/40540593/
Erfarenhetsberoende flexibilitet i ett molekylärt diversifierat centralt-till-perifert auditivt feedbacksystem – PubMed
https://pubmed.ncbi.nlm.nih.gov/36876911/
Laterala olivokokleära neuroner modulerar cochleära reaktioner på bullerexponering – PubMed
https://pubmed.ncbi.nlm.nih.gov/39854232/
Olivolar cochlear – Sök på Google
https://www.google.com/search?q=olivolar+cochlear&oq=&gs_lcrp=EgZjaHJvbWUqCQgAEEUYOxjCAzIJCAAQRRg7GMIDMgkIARBFGDsYwgMyCQgCEEUYOxjCAzIJCAMQRRg7GMIDMgkIBBBFGDsYwgMyCQgFEEUYOxjCAzIJCAYQRRg7GMIDMgkIBxBFGDsYwgMyCQgIEEUYOxjCAzIJCAkQRRg7GMIDMgkIChBFGDsYwgMyCQgLEEUYOxjCAzIJCAwQRRg7GMIDMgkIDRBFGDsYwgMyCQgOEEUYOxjCA9IBBi0xajBqN6gCD7ACAQ&client=ms-android-samsung-ss&sourceid=chrome-mobile&ie=UTF-8
Centrala kretsar och funktion hos de cochlea-efferenta systemen – PubMed
https://pubmed.ncbi.nlm.nih.gov/35606211/